No other heart rate monitor was specifically designed for newborn babies – they are all adult approaches that have been adapted for newborns. SurePulse VS is therefore, the first.
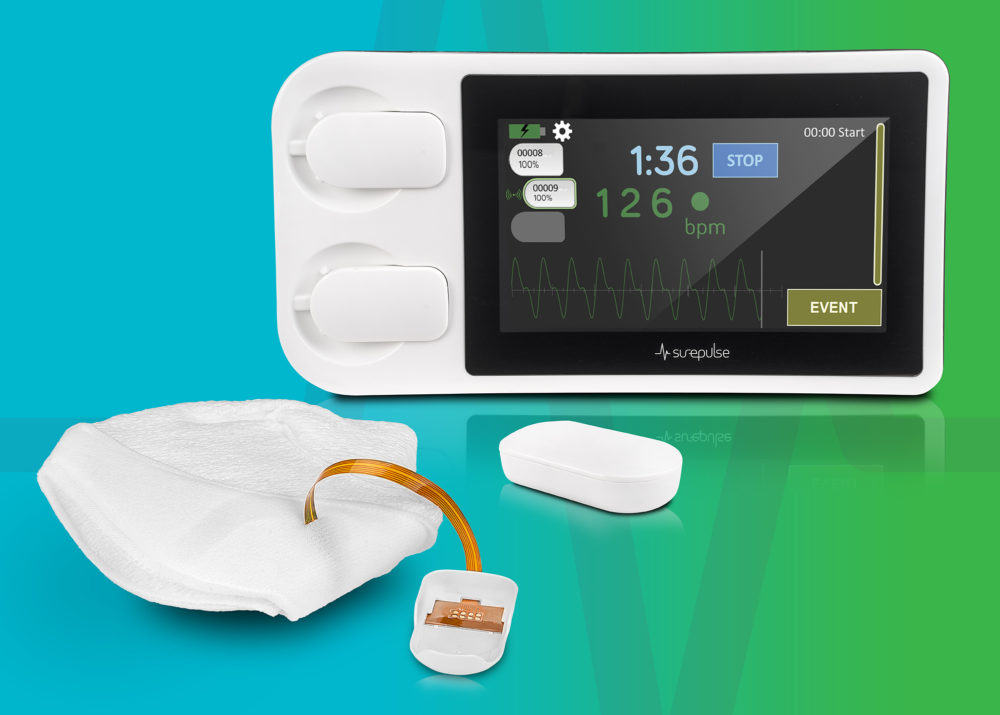
SurePulse VS facilitates confident decision-making – accurate and reliable heart rate information is displayed in realtime to the whole clinical team.
The heart rate of the baby is critical for guiding the stabilisation process and resuscitation interventions, as is recognised in National and International Guidelines. (Wyllie et al. 2015) However all current heart rate assessment approaches have drawbacks as in the critical first few minutes they do not always provide an early enough or sometimes an accurate enough heart rate.
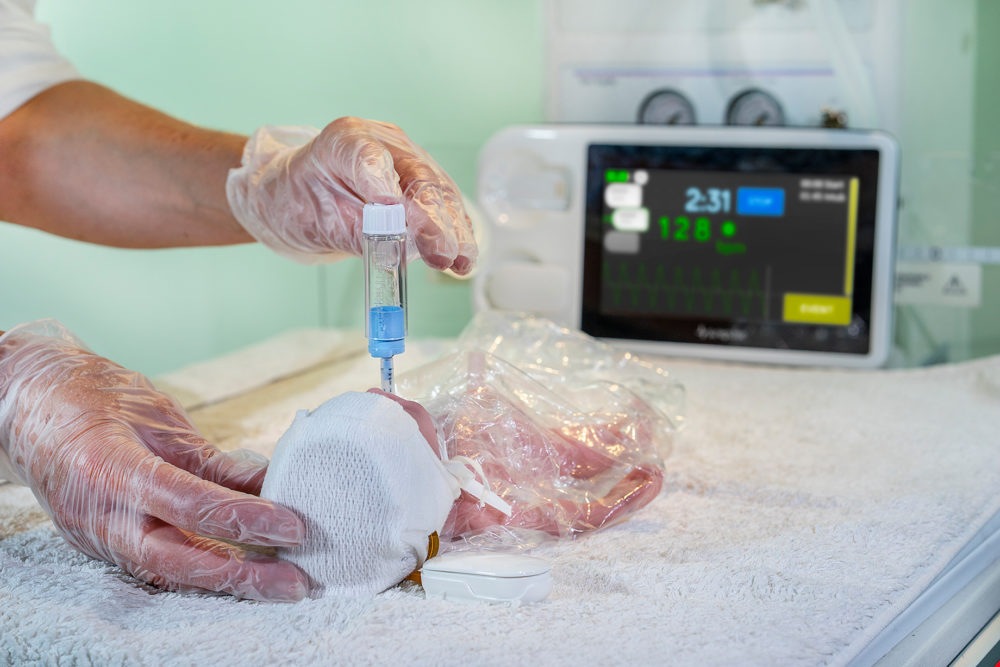
The device is wireless which provides significant opportunities for more flexible, evolving, delivery room practices (whilst monitoring heart rate). These include delayed cord clamping, skin-to-skin care (delivery room cuddles), early breast feeds and less-invasive approaches to stabilization

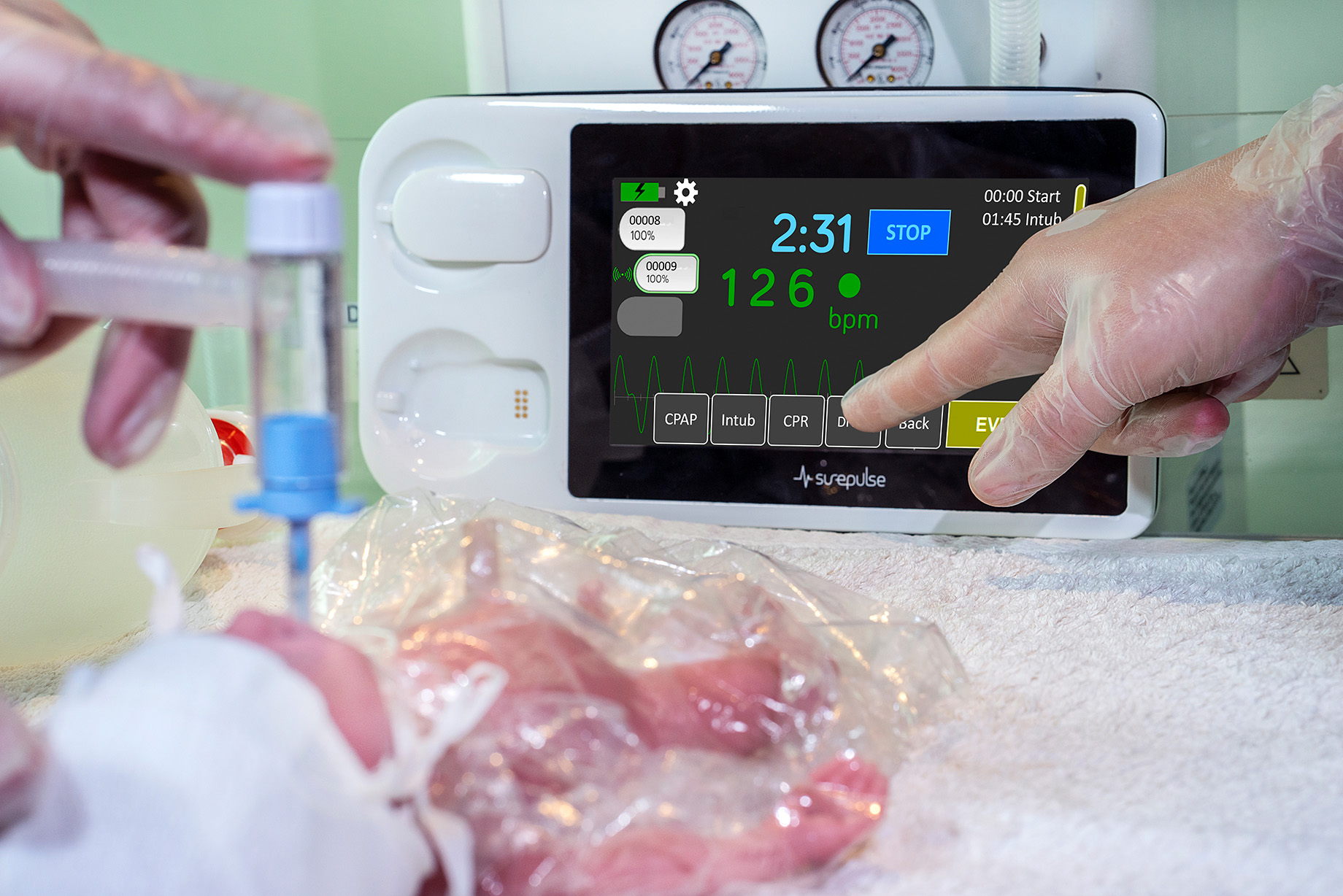
The touchscreen and data download facility supports improves patient safety, audit, clinical governance and training. By facilitating a date/time stamped downloadable record of heart rate information and the sequencing of clinical interventions, SurePulse VS supports the review of Delivery Room experience.
This also addresses a common problem as the recording of heart rate information and clinical interventions is notoriously inconsistent in the delivery room. The SurePulse VS touchscreen interface makes this easy and routine, and may also help if a retrospective review is needed – for example in a Serious Incident Investigation.
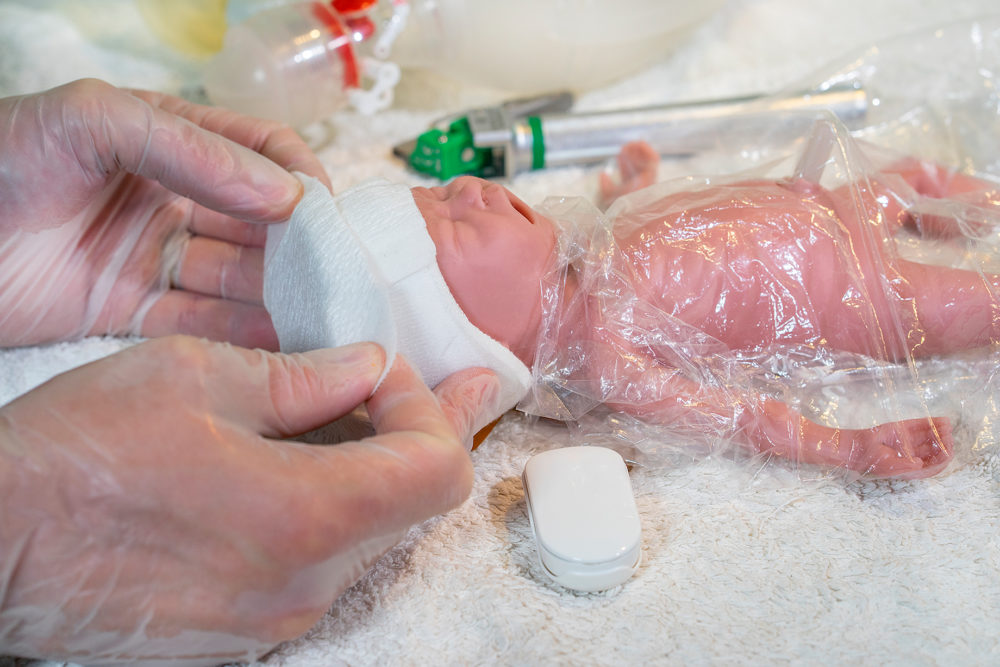
Many babies have a hand-knitted or medical cap put on their heads straight away to prevent heat-loss. As the SurePulse VS heart rate sensor is fitted into a very easy-to-fit cap then little process change needs to be made.
Clinical trials conducted at the Nottingham Universities NHS Trust established the thermoprotective equivalence of the SurePulse Cap, compared to other commonly used caps.

- Displays heart rate and pulse waveform continuously
- Touchscreen enables birth timer and event recording
- Battery life 4 hours (Modules 2 x 2 hours each)
- Award-winning design (Pickup et al)

- Carry-handle hooks over resuscitation table
- Heart rate and time/date stamped event data storage (100 hours)
- Data downloadable on datastick
- Reports generated as pdf and csv files
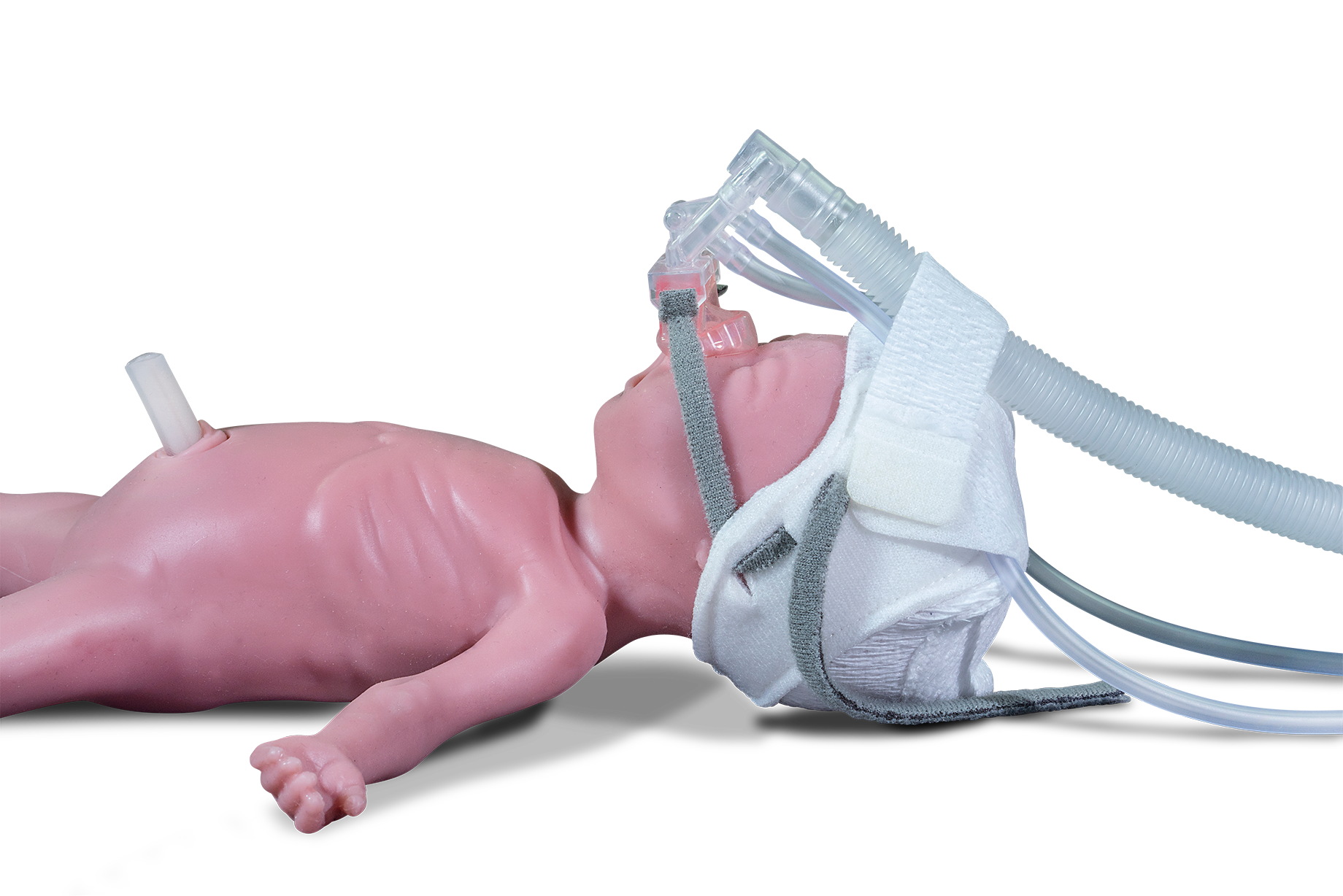
- Thermoprotective Cap in 5 sizes (23 weeks GA to 42 weeks)
- Easy to fit T-design
- Cap accommodates CPAP & endotracheal tube holders
There are significant potential economic and resource savings for healthcare systems if SurePulse VS is deployed clinically. In addition to the significant clinical and emotional consequences, the short and long term cost implications of poor resuscitation management are substantial as a lifetime of disability (as with Cerebral Palsy) carries a huge economic and resource burden. The consequences of overtreatment can also be considerable as unnecessary clinical interventions (for example if a stethoscope-assessed heart rate is underestimated and intubation or chest compressions are indicated) are associated with prolonged hospital stays in expensive Intensive Care facilities.

SurePulse VS provides the right heart rate information at the right time, and therefore will potentially improve adherence to guidelines and outcomes, and therefore has the potential to save healthcare systems many millions of pounds each year.
Request Business Case report and downloads
The SurePulse VS is a wireless heart rate monitor specifically designed for newborn babies. It displays this information, the pulse waveform and digital timer on the dedicated Display Unit, and also records this information as a downloadable record.
SurePulse VS uses reflective photoplethysmography (PPG) which is a similar technology to a pulse oximeter, but it utilises green light. The sensor is integrated into a thermoprotective Cap which is placed on the baby’s head at birth. The heart rate information is wirelessly sent to the Display Unit.
SurePulse VS provides wireless, accurate and continuous heart rate information (to within 5 beats per minute of ECG)
The Cap can be kept on the baby’s head (once the sensor has been removed) for 30 days. We recommend that the sensor is only kept in direct contact with the baby’s head for 4 hours – the sensor can be removed from the Cap without removing the Cap from the baby’s head, thereby minimizing heat loss
The Cap/sensor unit is single-use, and should be disposed of following local hospital guidelines and procedures. Due to the possibilities of contamination re-use of the Cap and sensor is not recommended
The Module is not disposable and should be returned to its dock in the Display Unit for re-charging after the monitoring session has ended
Once fully charged the Display Unit battery will last in use for 4 hours (the device can also be used whilst on mains power supply); each Module has, from full charge, a 2 hour battery life in use.
Clinical trials conducted at the Nottingham Universities NHS Trust are complete and have been submitted for publication in peer-reviewed journal; Clinical evaluation is currently taking place in several hospitals in Europe
Publications
M R Grubb et al 2014 Physiol. Meas. 35 881: ‘Forehead reflectance photoplethysmography to monitor heart rate: preliminary results from neonatal patients’ (Link to publications page)
L Pickup et al 2019 JMIR Hum Factors; 6(2): e12055.”The development of a clinical interface for a novel newborn resuscitation device; a Human Factors approach to understand cognitive user requirements.” (Link to publications page)
There are 5 Cap sizes which will accommodate babies from 20cm head circumference up to 40cm. This will normally mean that babies 23 to 42 weeks Gestational Age will be able to use the SurePulse VS device
The device records the heart rate and pulse waveform information and also has a touchscreen that can be used to record clinical events which are time and date stamped. This information can be downloaded as a permanent record and used for audit, training and clinical governance.

