PRESS RELEASE
We are delighted to announce that SurePulse VS has now been awarded CE certification.
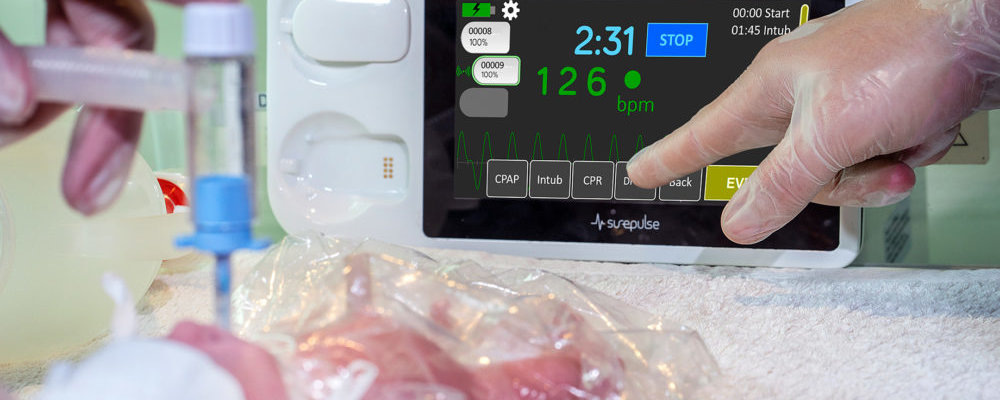
SurePulse VS represents a significant advance for babies, parents and clinicians in providing important and timely information to provide optimal care for newborn babies.
Amazingly, approximately 10% of newborns (80,000/yr in UK, 14 million/yr worldwide) require some form of enhanced support or resuscitation at birth. Reasons for this include being born too early or with other complications such as partial umbilical cord strangulation or congenital abnormalities. Those who receive the necessary aid quickly and efficiently will generally go on to lead normal lives, however those for which this is not the case are at risk of harm include damage to their brain or blindness for example.
International guidelines recognise that the baby’s heart rate is a key indicator of how well the baby is doing and how well interventions are working, but currently there is no reliable way of measuring heart rate straight after birth. A stethoscope is the most common heart rate assessment method, but is recognised as being prone to error.
SurePulse Medical Ltd was set up to solve this dilemma. The heart rate monitor that we have developed, SurePulse VS, has now received CE approval. It uses a small and safe optical sensor mounted in a soft cap placed on the baby’s head, which also helps to keep the baby warm. The SurePulse VS monitor has been successfully tested at the University of Nottingham NHS Trust Hospitals.
SurePulse Medical Ltd is a joint venture between the University of Nottingham and Tioga Ltd. This collaboration combines the academic strength of the University with the commercial and manufacturing capabilities of one of the UK’s premier Contract Electronics Manufacturers.
SurePulse VS is therefore now available to clinicians for heart rate monitoring in newborn babies throughout Europe.
Please direct any enquiries to info@surepulsemedical.com or telephone us on 0333 577 1133.
Editor’s Notes
- Background (clinical need):
Approximately 10% of newborn babies need assistance with their breathing at birth (that’s 80,000 per year in the UK, 400,000 in the US, 500,000 in the EU, and approximately 14 million globally). They are born not breathing and need support transitioning from the womb to the outside world. Heart rate is essential in guiding optimal care in these critical first few minutes of life. However there is considerable physician dissatisfaction with current offerings, as Stethoscope, Pulse Oximetry and ECG often do not provide an early enough or sometimes an accurate heart rate at this time. This can lead to uncertainty in how to manage any interventions optimally. For example, the difference between a heart rate of 65 and 55 beats per minute could mean the difference between initiating chest compressions or not, a significant intervention in fragile premature babies.
- Background (SurePulse Medical Ltd):
SurePulse Medical Ltd is a joint venture between the University of Nottingham (UoN) and the highly successful East Midlands based Contract Electronics Manufacturer Tioga Ltd, and was formed to commercialise patented technology developed at the UoN. SurePulse PPG technology has been utilised in over 340 babies at the Nottingham University Hospitals (including the Queen’s Medical Centre), including a 120-baby formal clinical trial.
SurePulse Medical Ltd are winners of the 2018 East Midlands Medilink ‘Start-Up’ Business Award and also winners of a place on the prestigious TMCx7 Texas Medical Centre Accelerator Programme.
For more information:
Website: www.surepulsemedical.com
Email: info@surepulsemedical.com
Tel: 0333 577 1133
ENDS