SurePulse VS
Research 0pportunities
Clinical and physiological
The SurePulse VS has additional neonatal data recording and output capabilities. This opens up exciting new research opportunities in neonatal physiology and clinical practice.
Clinical research opportunities
“There were more wires than him” (Bonner)
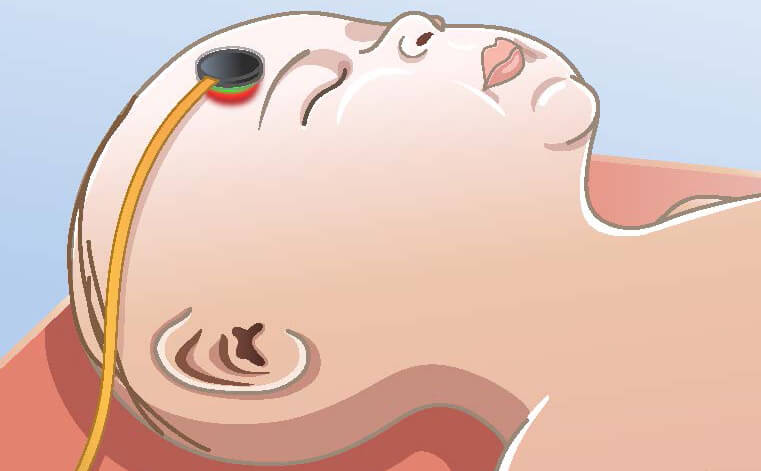
Delivery room practices are evolving at an unprecedented rate creating opportunities for research to provide evidence of clinical benefit to newborn babies. SurePulse VS is the first wireless forehead-mounted photoplethsymography (PPG) monitor for newborn babies and is available to support new areas of evolving research including delayed cord clamping, skin-to-skin (kangaroo care), delivery room cuddles, early breastfeeds, palliative care, and less-invasive approaches to stabilisation.
Delayed cord clamping
National and international guidelines are changing frequently on optimal cord management, depending upon the gestational age and health status of the baby.
The SurePulse VS research device provides recording and download of green, red and infrared waveforms which can be used to calculate oxygen saturation (real-time display, not CE approved) with the potential to enhance respiratory support, delayed cord clamping and less-invasive physiological approaches to stabilisation and resuscitation.

Wireless monitoring opportunities
Parents, and healthcare staff, often feel inhibited in engaging with their newborn babies in the delivery room (skin-to-skin care) or in intensive care (kangaroo care) on account of the plethora of monitoring wires. SurePulse VS offers new opportunities in enhancing family integrated care and can support wireless heart rate monitoring facilitating early cuddles, breastfeeds, and palliative care.
Physiological research opportunities
There are many exciting research opportunities to investigate the use of photoplethysmography (PPG) in newborn babies. The following areas of research are all underpinned by independent publications. The opportunities described here are not exhaustive and more research areas are being explored as the number of devices on the market increases.
3 wavelength reflection mode PPG
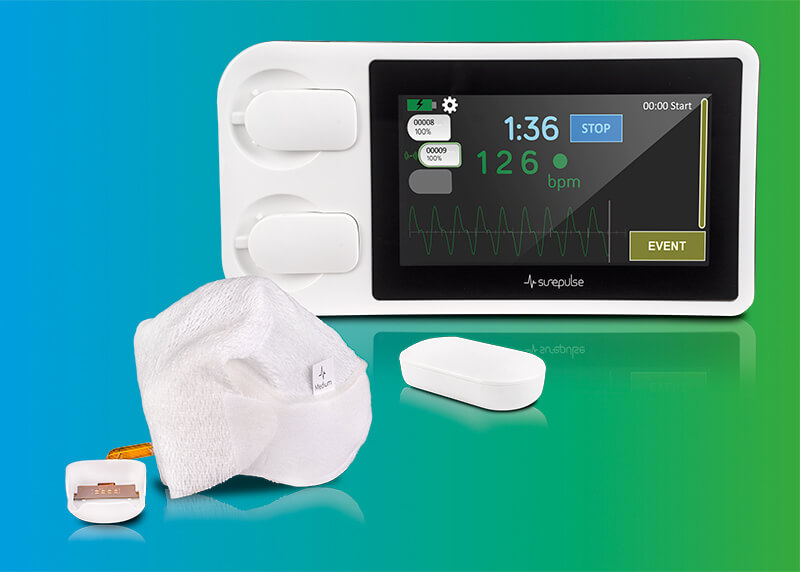
SurePulse VS (CE approved) provides outputs of green, red and infrared waveforms from the sensor, and several other outputs from the device including ambient light capture, LED brightness, event/intervention log, and technical performance data. Important physiological questions may be investigated – for example, blood oxygenation at birth is a critical indicator of clinical interventions and is currently a challenge to assess accurately. SurePulse VS allows the offline calculation of oxygen saturation (not CE approved for clinical use).
Two sensor options
The SurePulse VS is available in two forms, the single-use cap or the sensor alone fixed with tape. Both communicate wirelessly to the display monitor via the module. This facilitates further research into the optimal site for PPG monitoring. 100-hours of data can be stored and downloaded in CSV (Excel) and PDF formats.
Blood oxygenation changes after birth

Blood oxygenation changes in the first few minutes of a baby’s life are critical to understand to provide optimal care, yet remain a challenge in the delivery room. The wireless SurePulse VS can provide green/red/infrared trend data offline which may help answer important clinical questions such as FiO2 concentration and timing, optimal cord clamping timing, and minimally invasive transition monitoring.
Expanding neonatal research
The raw three-wavelength PPG from the SurePulse VS sensors facilitates the calculation of beat-to-beat pulse rate, pulse rate variability, pulse transit time, respiration rate, perfusion index tracking, oxygen saturation tracking, cardiac output and blood pressure derivatives (through calibration or calculation – analysis tools are available if required). These variables are experimental in nature and not CE approved for clinical use.