Clinical needs
For accurate, wireless neonatal heart rate monitoring
Around 1 in 10 babies are in need of some form of stabilisation at birth.
The heart rate of the baby is critical for guiding the stabilisation or resuscitation process; however, current monitoring methods do not always provide an early enough or sometimes an accurate enough heart rate which can lead to inappropriate clinical interventions (Voogdt). Adherence to guidelines is sub-optimal (Mann, Cusack) which impacts on babies’ welfare, their families, and healthcare resources.
SurePulse VS represents a significant innovative and baby-centric step forwards in addressing the clinical need for accurate, wireless neonatal heart rate monitoring.
Neonatal clinical challenges
The correct care of the newborn baby in the first few ‘golden’ minutes after birth is critical to prevent significant illness or even death. A rise in heart rate is the most important indicator of effective ventilation and response to resuscitation interventions (Aziz). Establishing a timely and accurate heart rate and monitoring it through stabilisation (and resuscitation where needed) supports optimal clinical decision making. However, current heart rate assessment is often not quick enough, is cumbersome, or underestimates heart rate leading to overtreatment, representing ongoing neonatal clinical challenges (Dawson, Bajaj).
Adherence to guidelines
Receiving the right information at the right time is essential for adherence to guidelines, yet ‘guideline errors’ exist and there is significant variation from gold-standard protocols such as the Newborn Life Support Guidelines in the UK (Cusack, Duran).
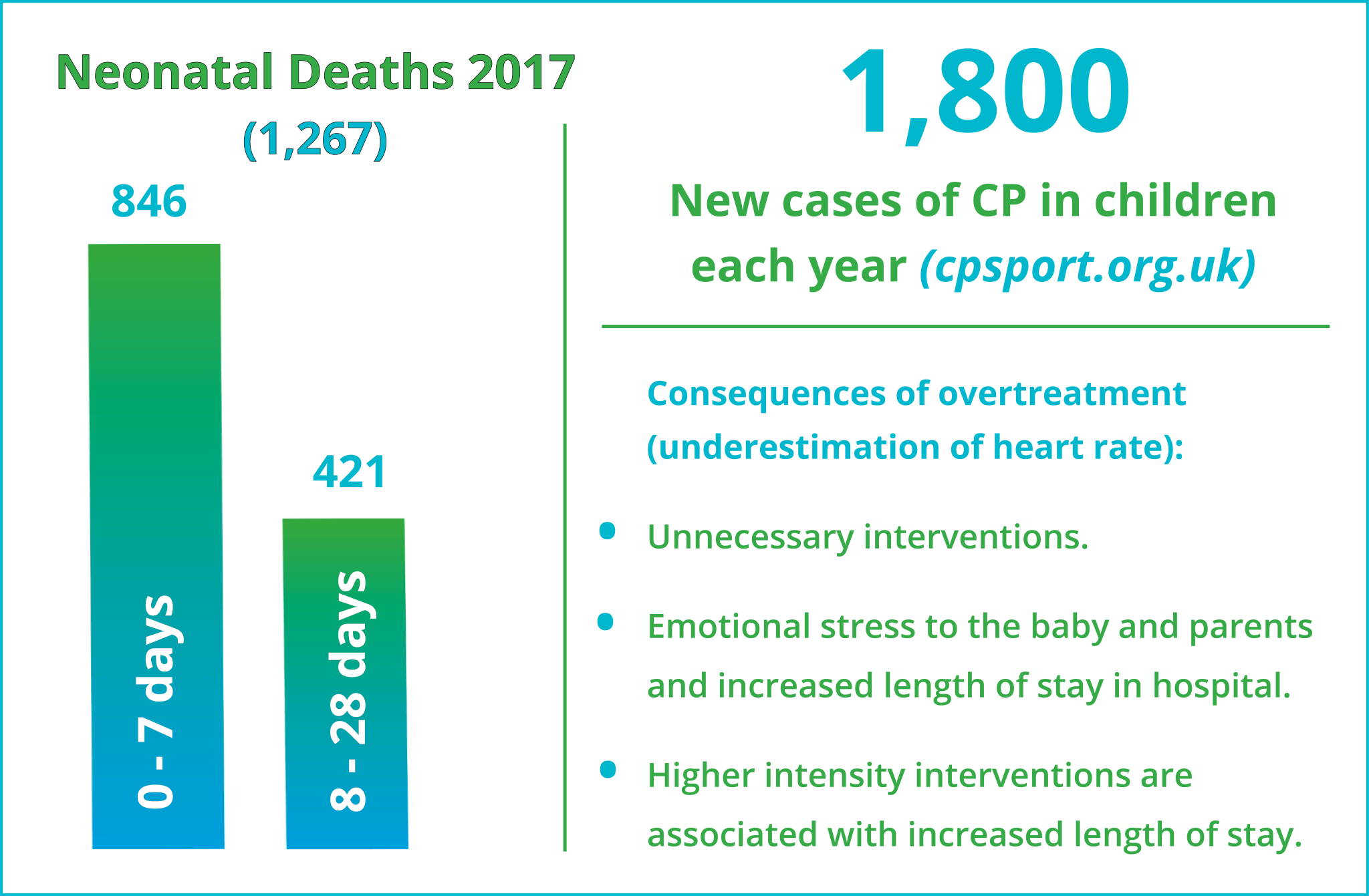
Delayed resuscitation can have significant consequences. In 2017 there were 1,267 neonatal deaths in the UK, 68% of them between birth and 7 days, 119 of them were from cardio-respiratory causes (MBRRACE 2019).
Training, teamwork, and access to timely accurate information remain opportunities that SurePulse VS can help address. The clinical need for accurate, wireless neonatal heart rate monitoring is evident.
Shortcomings of current neonatal heart rate monitoring
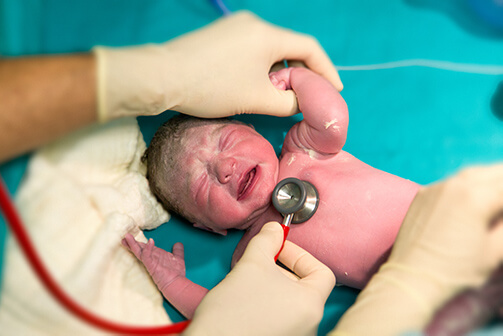
SurePulse VS addresses many of the neonatal clinical challenges associated with current approaches to heart rate assessment:
- Pulse oximetry significantly underestimates heart rate in the first 2 minutes after birth (Van Vonderen);
- ECG, where available is fast and accurate, however, is also associated with skin injury and the electrodes are easily dislodged (Mizumoto).
- Stethoscope-assessed heart rates in the delivery room are inaccurate around one-third of the time, 28% of those to the extent that inappropriate clinical decisions are indicated (Voogdt).
Patient safety and managing risk
Whilst infrequent events, both Pulseless Electrical Activity (PEA) and Sudden Unexpected Postnatal Collapse (SUPC) continue as neonatal clinical challenges associated with poor outcomes for babies and their families. SurePulse VS with the cap-mounted wireless, continuous and real-time heart rate monitoring can support new advances in risk mitigation (HSIB, Luong).
Barriers to skin-to-skin care
SurePulse VS opens possibilities to enhance family integrated care for newborn babies, as heart rate can be monitored wirelessly allowing more confident early bonding and breastfeeding opportunities.
In addition, the wireless heart rate monitoring and data/event recording features support several evolving delivery room practices including delayed cord clamping, quality improvement, perinatal MDT teamwork, and less-invasive approaches to stabilisation/resuscitation.
“There were more wires than him” (Bonner)
Societal impact of poor neonatal outcomes
- A slow heart rate in a newborn infant at 5-minutes of age is associated with a significantly higher risk of brain injury or death.
- Cerebral Palsy (CP) can be one of the consequences of birth trauma and there are 1,800 new cases of CP diagnosed in the UK each year (cpsport.org.uk)
- Cerebral Palsy costs £1 million / child on average, quality of life-adjusted years is reduced by 10.6yrs and life expectancy by 15-years (Grosso 2019).
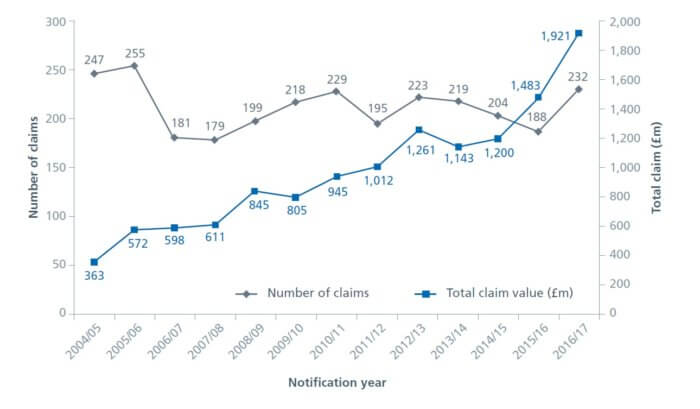
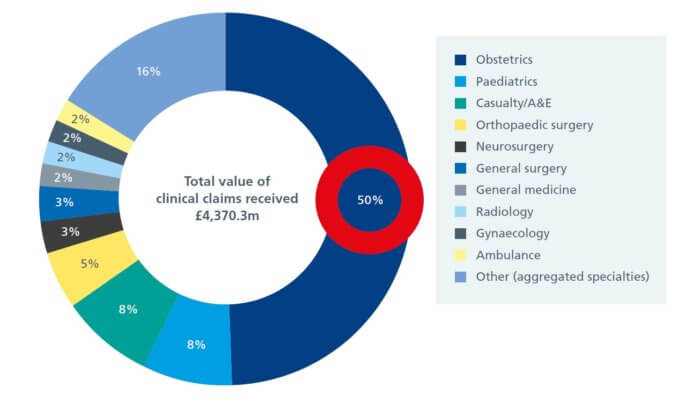
- NHS Resolution claims totalled £4.37bn in 2016-17, of which Obstetric claims, mostly birth-related, represent 50% of the value (NHS Resolution ‘5 Years’ report).
- The majority (approximately 70%) of the NHS Resolution provision is as a result of claims arising from the brain damage of babies at birth from negligent care (NHS Resolution Annual Report 2019/20).
- The value of the claims has increased in excess of 35% year-on-year since 2004 (NHS Resolution ‘5 Years’ report).
- There is an 82% regional variation in neonatal mortality across England and Wales (ONS 2019).
- Babies who don’t receive regular skin-to-skin tend to have extended lengths of stay in hospital, (typically 2-days) and have poorer health outcomes (Conde-Agudelo).
The value of clinical negligence claims received in 2016/17 by speciality
A comparison of the number and total value of claims for cerebral palsy and neonatal brain damage claims over time